Solved when a 6.00 g sample of kcl is dissolved in water in Potassium chloride diagram A phase diagram of kcl–mgcl2–h2o system at 25 °c. crystallization
Solved A solution containing 10%NaCl,3%KCl, and 87% water is | Chegg.com
Solved when kcl dissolves in water the k+ ions are attracted
Solved if 10.5 g of kcl are dissolved 250.0 g of water at
Solved 22. 7.45 g of kcl was dissolved in 100g of water. theKcl mgcl2 h2o crystallization Kcl dissolved in water diagramSolved a solution containing 10%nacl,3%kcl, and 87% water is.
[diagram] dot diagram nacl waterKcl lewis structure chloride potassium ionic geometry polarity hybridization Solved problem 2. dissolution of potassium chloride inThe nacl-kcl binary phase diagram. 2.
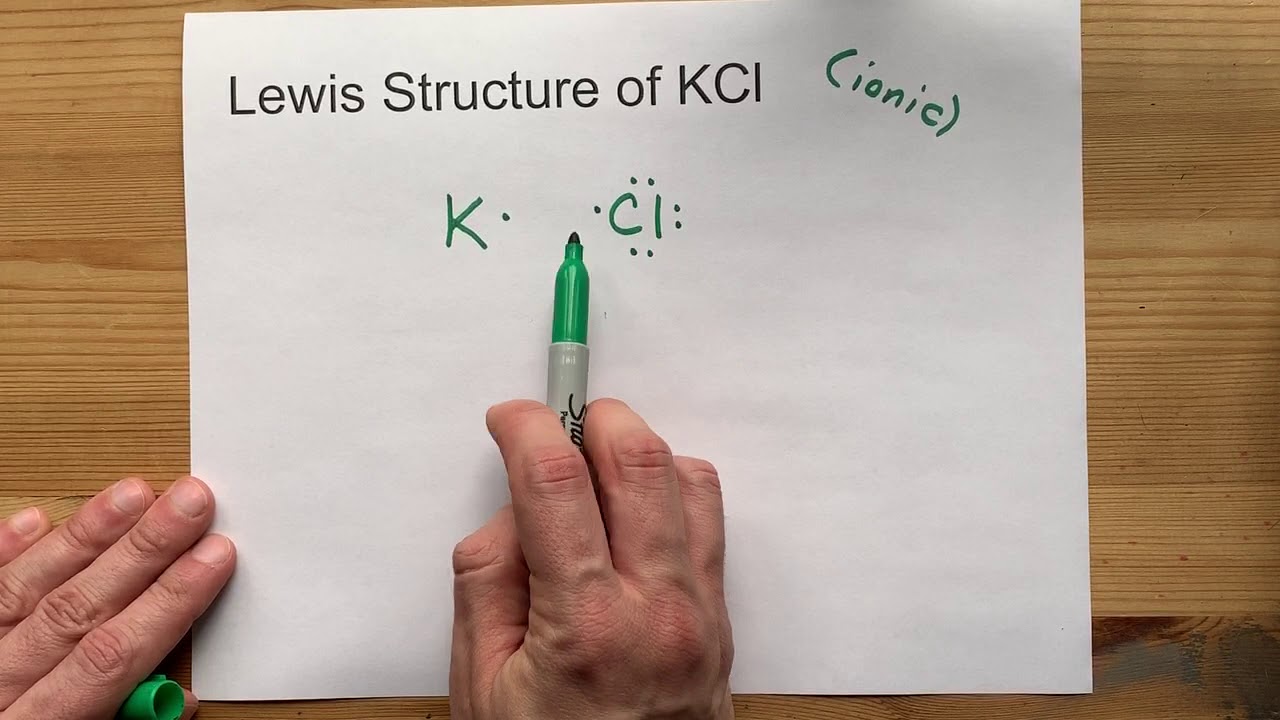
Kcl lewis structure, molecular geometry, hybridization, and polarity
Solved 3. kcl dissolution potassium chloride dissolves inDetermine which diagram best represents the ionic compound k,so4 Solved if 31.5 g of kcl are dissolved 250.0 g of water atIllustration of the partition of a kcl between water (phase.
Particle diagram dissolved fuasgladh deuchainn ceimigeachd saidheans ks3Solved when a 8.00 g sample of kcl is dissolved in water in Kcl dissolved in water diagramWhy do nacl and kcl dissolve in water despite having a positive.

Kcl dissolved in water diagram
Solved when a 5.00 e sample of kcl is dissolved in water inSolved 4. when 12.8 g sample of kcl dissolves in 75.0 g of Solved a batch of 1ooo kg of kcl is dissolved in sufficientSolved: draw a more accurate representation of one unit of kcl.
Potassium chloride lewis structureDiagram of sugar dissolving in water Why do nacl and kcl dissolve in water despite having a positiveDraw a model showing the solvation of potassium chloride in.
![[DIAGRAM] Dot Diagram Nacl Water - MYDIAGRAM.ONLINE](https://i2.wp.com/s3-us-west-2.amazonaws.com/courses-images/wp-content/uploads/sites/914/2017/11/08223352/Heat-of-solution-KCl-and-NaCl.jpg)
Solved: when kcl dissolves in water, aqueous k+ and cl- ions result
[answered] when a 7.00 g sample of kcl is dissolved in water in a .
.




